Therapeutic Considerations for Horses Presenting Lameness From Palmar Foot Pain Reprinted with permission from the American Association of Equine Practitioners.
Originally printed in the 2006 AAEP Convention proceedings G. Kent Carter; and Robin Dabareiner Authors' address: Department of Large Animal Clinical Sciences, Texas A&M University, College Station, TX 77843; e-mail: . © 2006 AAEP. 1. Introduction Managing horses with posterior foot pain is one of the most common and problematic types of lamenesses in which we deal. Lame horses are usually considered to have the source of pain originating from this area of the foot based on a combination of physical examination findings and response to diagnostic analgesia. However, recent studies have shown that considerable overlap exists between diagnostic nerve and joint blocks,1,2 and many diagnostic nerve blocks lack the specificity that we previously assumed existed.3 Although the potential exists for confusing results and inaccurate interpretation of the response to these nerve blocks,4 we recognize these limitations. We still rely on them in conjunction with our physical examination findings to help localize the source of pain within the foot. For example, lameness originating from the cranial aspect of the foot (toe region) can also be blocked by both a palmar digital nerve (PDN) block2 or a distal interphalangeal (DIP) joint block,5 but we usually trust our physical examination and other diagnostic tools such as hoof-tester response and radiographic findings to rule out lameness originating from this region. We generally feel confident that the source of pain and lameness originates from somewhere in the posterior aspect of the foot when the horse responds favorably to a properly applied PDN,6 DIP joint, or navicular bursa (NB) block and when we lack clinical exam findings to suggest otherwise. After determining, to the best of our ability, that the lameness is originating from the posterior onehalf of the foot, we must then determine the best therapeutic option suited to that particular horse. It is this author's opinion that the specific diagnosis in each individual case guides the appropriate therapy rather than categorically grouping all cases into one classification such as "heel syndrome" and treating them as one disease process. For example, subsolar/frog bruising, navicular disease, and distal deep flexor tendonitis have similar lameness examination findings and response to diagnostic analgesia, but each would warrant a different therapeutic approach. Recent advancements in imaging capabilities have significantly changed our knowledge about lameness originating from the foot3,7,8 and subsequently, has changed our thinking about appropriate therapeutic options. Magnetic resonance imaging (MRI), computed tomography (CT), and ultrasonography have identified a population of footsore horses that have soft-tissue injuries that were not previously recognized.3,7-9 Before our recognition of these soft-tissue injuries, many of these horses were inappropriately treated as though they were "navicular syndrome" horses without radiographically apparent lesions. Although MRI and CT are new and exciting imaging modalities, the fact remains that they are not "lameness machines" that provide all the answers. However, they do provide valuable additional information when used in conjunction with our physical examination findings and other traditional imaging modalities such as radiology, ultrasound, and nuclear scintigraphy. This new information has unveiled a whole new group of horses that warrant a new (and sometimes old) approach to therapy and convalescence. The use of MRI and CT is becoming readily more available to equine practitioners, but the fact remains that, in many cases, this new information is still not used because of financial constraints or lack of availability. Therefore, many lamenesses originating from the foot do not get "exactly" diagnosed with a demonstrable pathologic lesion. In the future, we can hopefully learn from the cases that do get an exact pathologic lesion identified and can then apply these finding, where appropriate, to other similar clinical cases. 2. Therapeutic Approaches It is not within the scope of this paper to describe our diagnostic approach to the sore-footed horse; however, our opinion is that the therapy is dictated by the specific diagnosis obtained. When a definitive diagnosis of the pathologic lesion cannot be obtained, then the most appropriate therapy for the abnormalities identified is indicated. We consider all aspects of the examination when considering the best therapeutic approach for the case at hand. History is an important aspect of the examination, and lamenesses with an acute versus insidious onsets are usually approached differently. An acute onset grade IV/V lameness limited to one front foot would warrant different therapeutic considerations than a chronic, insidious onset, bilateral front foot lameness. Physical examination finding such as response to digital palpation and hoof-tester (HT) evaluation can be extremely important in helping to determine a therapeutic approach. Despite irregularities to HT response and occasional confusing results, we feel that a thorough, systematic HT evaluation can be one of the most useful diagnostic tools of the lameness examination. Hoof testing is often useful in determining if pain is originating from the hoof capsule, heels, or sole versus the more central aspects of the foot. Therapy for a horse that hoof tests positively across the heel and bar (like heel bruising) would require a different therapeutic approach than would a horse presenting pain more consistent with navicular disease. Response to diagnostic analgesia is not without limitation, and the potential for confusing results exists.4 However, in our opinion, it is invaluable in trying to arrive at the most definitive diagnosis possible. Diagnostic imaging is often used to arrive at the most definitive diagnosis possible. The mode of imaging applied will vary with the case, but radiography is routinely and most often applied to cases. Ultrasound, nuclear scintigraphy, MRI,and CT are applied in selected cases, and all have their appropriate applications The results of the clinical evaluation and imaging will sometimes arrive at a specific diagnosis that leads to a subsequent therapeutic plan. However, there are a number of cases where a definitive diagnosis, as to which structure within the foot is affected, cannot be obtained for some reason. In these cases, therapy must be initiated based on the clinician's most likely diagnosis as well as clinical impressions and past experiences. In these cases, we try to localize and rationalize the most appropriate diagnosis from all the data (e.g., history, physical examination, HT examinatiopn results, response to diagnostic analgesia, and diagnostic imaging) and initiate therapy accordingly. In cases where a definitive diagnosis is unknown, we try to determine, to the best of our ability, if shoeing and hoof balance may be related to the cause of pain. We also try to determine if the lameness originates from the hoof capsule, laminae, sole, or subsolar corium. We try to determine if the lameness seems to be related to a soft-tissue injury in the more central aspect of the foot, such as the distal deep flexor tendon, impar ligament, or suspensory ligament of the navicular bone, or if the horse seems to have progressive degenerative navicular disease. It is this author's opinion that shoeing and hoof/limb balance is an extremely important aspect of every foot lameness. In every lameness originating from the foot, the role of shoeing and hoof balance should be considered in the etiology and possible therapy of the lameness. In some cases, such as heel bruises, corrective trimming and shoeing is the indicated therapy. In many cases, such as softtissue injuries, correct trimming and shoeing are extremely important as adjuncts to other therapeutic modalities. Although many horses remain sound with poor shoeing and hoof care, there are many lamenesses either initiated or exacerbated by poor-quality hoof care. A percentage of horses with foot lameness can be effectively cured or successfully managed with appropriate hoof care alone. There is also a population of lame horses in which appropriate shoeing and hoof care is beneficial for the long-term management of the lameness, but additional therapies are required for athletic use. Another lesser population of horses with excellent foot care, balance, and hoof structure remain lame despite all therapy. It has been our experience that corrective or "correct" shoeing and trimming is most successful in those horses with structurally sound but poorly managed feet. It is not in the scope of this paper to fully discuss shoeing options for lameness originating from the foot, but it will be discussed at length in another paper in this series; however, observation of hoof balance, structure, and shoeing is an integral component of every foot lame horse. The importance of athletic rest and a convalescent period are often overlooked in horses that have lameness originating from the feet. In horses with a diagnosed or presumed soft-tissue injury, controlled exercise or removal from athletic performance can be an extremely important aspect of case management. Simply removing their shoes and turning them out for several months is rarely curative, and a more controlled approach to exercise and return to use is preferable in conjunction with other initiated therapies. It is our experience that uncontrolled pasture turnout can be traumatic to healing soft-tissue injuries, and a more controlled progressive exercise regime is usually recommended. Intrasynovial injections of anti-inflammatory agents such as hyaluronic acid (HA) and corticosteroids are commonly employed in the therapeutic management of lameness originating from within the foot. Historically, lameness that responded favorably to diagnostic anesthesia of the DIP joint was assumed to originate from the joint itself, and subsequently, coffin-joint medication with anti-inflammatory agents became commonplace.10 Previous studies in which dye11 or anesthetic solutions12 were injected into the coffin joint showed that diffusion of the solutions occurred from the DIP joint to the navicular area of the foot. Another study showed that diagnostic anesthetic solutions would diffuse from the DIP joint into the NB, which showed that pain originating from the navicular region of the foot, including the NB, could be alleviated by DIPjoint injection of local anesthetic.13 These studies and clinical experiences of improving foot pain with DIP-joint medication have lead to the common practice of injecting the coffin joint in horses with sore feet. However, there are a significant number of horses with foot pain that improve after a PDN block and/or DIP joint anesthesia yet fail to respond favorably to intra-articular DIP-joint injection of anti inflammatory agents. Recently, Dabareiner et al.14 reported on foot-lameness cases that blocked out to an intra-articular coffin-joint block but failed to respond favorably to coffin-joint therapy. These horses later responded favorably to NB injection of corticosteroids and HA.14 Although coffin-joint injections are easier to perform and less invasive than injection of the NB, we have found it to be less useful than NB medication in managing advanced navicular disease. We commonly use coffin-joint medication if we suspect that coffin-joint pain or disease is present or if there is mild navicular disease or softtissue inflammation. Coffin-joint medication is often tried as an initial treatment modality, but if the horse does not respond favorably to this treatment, then medication of the NB is performed. Over the years, we have injected a large number of DIP joints without significant side effects. Occasional joint inflammation or sepsis is reported, but we have not recognized subsequent joint deterioration as a result of our therapy. Treatment with intra-articular medications varies with clinician, severity of disease process, and client. We usually inject 20 mg of sodium hyaluronate and 3-6 mg of triamcinolone (Vetaloga) which, if helpful, will often alleviate clinical signs of lameness for 6-12 wk. In severe cases of navicular disease, we may use 20-40 mg of methylprednisonole acetate (Depo Medrolb) in combination with the sodium hyaluronate, because it may provide a slightly longer duration of effect. The sodium hyaluronate is used for joint lubrication and to increase the HA content of synovial fluid. Corticosteroids work through interaction with steroid-specific receptors in the cytoplasm of the cells and attenuate inflammation by inhibiting inflammatory infiltration into the joint. They also inhibit neutrophil function by impairing lysosomal enzymatic release. Corticosteroids also inhibit phospholipase A2, which blocks both cyclooxygenase and lipoxygenase inflammatory pathways. The two primary reasons not to use intra-articular corticosteroids in horses with joint disease are the risk of sepsis and the potential detrimental effects that the drugs may have on articular cartilage and subchondral bone. Using aseptic joint-injection techniques and combining intra-articular antimicrobials to the injection medication should decrease the risk of synovial infection. We prefer to use 50-100 mg of Amikacin sulfatec for joint injections. To minimize the negative effects of intra-articular corticosteroids, we try to use the lowest clinically effective dose possible. Minimal research has been performed in this area, so clinicians must rely on experience and empirical information. Both systemic and intra-articular corticosteroids have been associated with laminitis in horses, but we have never experienced this after intra-articular injections. NB injections have rapidly gained increased use in the maintenance of chronic foot lameness, particularly in Western performance horses (Fig. 1). When we feel we are treating navicular disease or soft-tissue inflammation in the posterior aspect of the foot such as a navicular bursitis, then medication of the NB is commonly employed to keep these horses athletically active. We usually inject 3-6 mg of triamcinilone (Vetalog) in combination with 10 mg of hyaluronic acid with the addition of 50 mg of Amikacin sulfate. When a significant soft-tissue injury is suspected, rest in conjunction with NB injections may be indicated. If significant flexor cortex lesions are radiographically evident on the navicular bone (Fig. 2), medication of the NB may allow continued use of the horse, but deep flexor tendonitis/rupture secondary to the cartilage erosion may occur. The owner should be cautioned about the possibility of this complication and the other dangers involved. 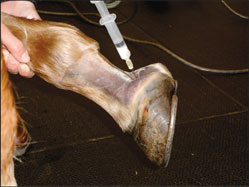 | | Fig. 1. Photograph of needle in navicular bursa. | Recently, as a result of deep flexor lesion findings discovered by MRI, treatment of the digital flexor tendon sheath with HA and corticosteroids has been used.15 The idea is to treat the tendon sheath that is in close proximity to the damaged tendon, which will decrease inflammation and improve healing. In addition, decreased lameness should allow continued exercise in a very controlled exercise program, and this may facilitate tendon healing. Intralesional injection into tendon lesions with substances intended to improve healing of damaged tendons has been described.16,17 Recently, CTguided injection of stem cells into distal deep flexor tendon lesion has been described and found to be beneficial in horses with chronic deep flexor injuries within the foot.9 Similarly, shock-wave therapy has been found to be beneficial to the healing of tendon injuries.18 These findings have lead to the use of shock-wave therapy for horses with suspected soft-tissue injuries within the foot and/or navicular disease. Long-term follow up reports are yet to be published about this treatment modality, but the results seen by this author have not been encouraging. 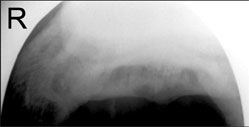 | | Fig. 2. Flexor view of navicular bone showing lysis on cortex. | 3. Oral Medications Isoxsuprine is a B-agonist that causes peripheral vasodilation in people. It has been used as an adjunct treatment for navicular disease has been in use for ~20 yr.19 Clinicians' opinions on the effectiveness of this therapy have been varied. More recent work on the isoxsuprine blood levels and effects20 do not support its use, because it has erratic absorption after oral administration in the horse. This author rarely recommends its use, but occasionally, cases are encountered where the owners feel isoxsuprine therapy is beneficial. Tiludronate is a drug licensed in Europe that inhibits the activity of the osteoclasts and thus, reduces osteolysis of the navicular bone; it has been reported to be potentially beneficial in the treating navicular disease.d It is currently not available for use in the United States, and this author has no experience using it. Systemic anti-inflammatory medications are often employed in the short- and long-term management of lameness originating from the foot. Phenylbutazone is most commonly selected in the treatment of navicular/heel pain, primarily because of the low cost and ease of application, which is especially important for long-term therapy. It reduces pain by inhibiting the enzyme cyclooxygenase and the subsequent cascade of prostaglandins. It also inhibits platelet aggregation that may theoretically increase blood flow to the foot. Phenylbutazone can be used to break the pain cycle and allows adjustment to new hoof angles and shoeing changes. In suspected soft-tissue injuries, we use 2 g one time daily for 7-10 days to control pain and decrease inflammation. It is some clinicians' preference to use flunixin meglumine for this short-term, high-dose antiinflammatory therapy. It is not uncommon for us to use phenylbutazone at a low dose (1 g daily) for the long term in horses with chronic foot lameness. We may use this dose indefinitely when the horse is in active competitions. Although the risk of gastric and right dorsal colon ulceration exists with chronic non-steroid anti-inflammatory therapy, we rarely see problems at this dose. 4. Systemic Hyaluronate If coffin joint or NB synovitis is suspected, adjunct therapy with IV Legende is often employed and may be beneficial. Systemic HA may be more effective when used on horses with mild synovitis/capsulitis and less effective on horses with chronic osteoarthritis. The recommended dose is 40 mg HA given 1 day/wk for 3 wk by IV; then, the dose is administered one day a month for maintenance. HA is a normal component of synovial fluid and functions as a joint lubricant. It also seems to have some antiinflammatory properties, but the exact mechanism is unknown. It has been shown that exogenous HA inhibits chemotaxis and phagocytosis of granulocytes; it also reduces the stimulation of lymphocytes and may decrease the formation of prostaglandin synthesis. The anti-inflammatory properties of HA seem to be dependant on the dose, and HA with a molecular weight >500,000 Da may be more effective.21 Previous clinical reports on the use of systemic HA in lame horses has been supportive.21 Polysulfated glycosaminoglycans (PSGAGs) such as Adequanf are referred to as chondroprotective agents and are used to prevent, attenuate, or reverse morphologic cartilaginous lesions associated with osteoarthritis. Adequan is made from bovine lung and trachea extracts that contain mostly chondroitin sulfate. Previous studies have shown that the anti-inflammatory effect of PSGAGs involve the inhibition of enzymes and cytokines associated with osteoarthritis such as interleukin -1, which is a potent chemotactant agent, metalloproteinases, and Prostaglandin E2 (PGE2). Both in vivo and in vitro equine studies have been performed with conflicting results. More recently, a survey of 1522 equine veterinarians assessing the efficacy of systemic PSGAGs resulted in the subjective conclusion that the use of PSGAG was more effective than HA for treatment of subacute degenerative joint disease and less effective for acute synovitis.22 Intramuscular PSGAG was shown to improve lameness in horses diagnosed with navicular syndrome in one double-blinded study. The dose was 500 mg IM every 4 days for eight treatments. Lameness improved when the horses were on therapy but reappeared after discontinuation of therapy. This study supports continuous therapy with PSGAG as an adjunct therapy for navicular disease.23 In our hospital, we see a large number of Western performance horses with chronic navicular disease. Intramuscular Adequan (500 mg) given every week seems to aid in the management of these cases; however, scientific studies do not support the change in frequency of its use. Oral chrondromodulatory nutraceuticals have been evaluated in the treatment of 10 horses with navicular syndrome. The nutraceutical consisted of 9 g of glucosamine, 3 g chondrotin sulfate, and 600 mg manganese ascorbate, and it was given orally twice daily for 60 days. The clinical impression from the owners was that the horses had an improvement in lameness.24 Recently, the ability of the horse to absorb these oral nutraceuticals has been questioned. Ten horses were given 8.0 or 16.0 kDa orally, which is the maintenance and double dose, respectively, of the Cosequing equine product that consists of both chondrotin sulfate and glucosamine. Disaccharides formed specifically from the breakdown of chondroitin sulfate, but not glucosamine, were found in the horse's plasma after oral dosing of an 8.0 and 16.9 kDa sample.25 These results suggest that chondroitin sulfate is absorbed after oral administration in the horse. We have found that evaluating its use in horses with navicular-area pain is very difficult. Some owners feel that their horses improve with daily administration, and some do not. Empirically, the benefit would seem to be inferior to systemic administration of IV HA or IM Adequan. 5. Surgical Options A palmar digital neurectomy is commonly employed in the management of chronic navicular disease or other posterior foot lamenesses that significantly improve after a PDN block. In our opinion, the use of a neurectomy for treatment of posterior foot pain has decreased significantly over the years. This is probably because of the complications encountered, such as neuroma formation, nerve regrowth, unrecognized sepsis of the foot, and deep flexor tendon rupture. Erosive flexor cortex lesions of the navicular bone (Fig. 2) pre-dispose to deep flexor tendon damage and potentially, catastrophic rupture. Therefore, this author is extremely cautious about having a palmar digital neurectomy performed on horses with posterior foot pain that have this radiographic lesion present. With the availability of MRI imaging, it seems prudent to evaluate the integrity of the deep flexor tendon before performing this surgical procedure. 6. Summary Treatment strategies for horses with lameness originating form the posterior foot depend on the diagnosis or presumed etiology for therapy to be successful. It is important to implement appropriate hoof care and shoeing to assist in the treatment. Many cases require a combination of corrective trimming/shoeing, intrasynovial medications, and controlled exercise/athletic rest regimes. The implementation of a period of relative rest with progressive return to full exercise with systemic anti-inflammatory medications can be beneficial in the long-term management of incurable chronic conditions such as chronic navicular disease. - Schumacher J, Schramme M, Schumacher J, et al. A review of recent studies concerning diagnostic analgesia of the equine forefoot, in Proceedings. 49th Annual American Association of Equine Practitioners Convention 2003;312-316.
- Easter JL, Watkins JP, Stephens SL, et al. Effects of regional anesthesia on experimentally induced coffin joint synovitis, in Proceedings. 46th Annual American Association of Equine Practitioners Convention 2000;214-216.
- Dyson S, Murray R, Schramme M, et al. Lameness in 46 horses associated with deep digital flexor tendonitis in the digit: diagnosis confirmed with magnetic resonance imaging. Equine Vet J 2003;35:681-690.
- Carter GK. Diagnostic anesthesia in the lameness examination: potential areas of confusion, in Proceedings. 51st Annual American Association of Equine Practitioners Convention 2005;1-5.
- Schumacher J, Steiger R, Schumacher J, et al. Effects of analgesia of the distal interphalangeal joint or palmar digital nerves on lameness caused by solar pain. Vet Surg 2000;29:54-60.
- Schumacher J, Livesey L, Schumacher M. Effect of anesthesia of the palmar digital nerves on lameness caused by pain in the proximal interphalangeal joint, in Proceedings. 46th Annual American Association of Equine Practitioners Convention 2000;214-216.
- Schramme M. A comparison between magnetic resonance imaging, pathology, and radiology in 34 limbs with navicular syndrome and 25 control limbs, in Proceedings. 51st Annual American Association of Equine Practitioners Convention 2005;348-358.
- Martinelli M. Relationship between nuclear scintigraphy and standing MRI in 30 horses with lameness of the foot, in Proceedings. 51st Annual American Association of Equine Practitioners Convention 2005;359-365.
- Puchalski SM. Contrast-enhanced computed tomography of the equine distal extremity, in Proceedings. 51st Annual American Association of Equine Practitioners Convention 2005;389-394.
- Goodman NL. Quarter Horse race track practice, in Proceedings. 33rd Annual American Association of Equine Practitioners Convention 1987;835-841.
- Bowker RM, Rockershouser SJ, Sonea IM, et al. Immunocytochemical and dye distribution studies of nerves potentially desensitized by injections into the distal interphalangeal joint or the navicular bursa of horses. J Am Vet Med Assoc 1993;203:1708-1714.
- Gough MR, Mayhew IG, Munroe GA. Diffusion of mepivacaine between synovial structures in the horse. Part 1: forelimb foot and carpus. Equine Vet J 2002;34:80-84.
- Plesant RS, Moll HD, Ley WB, et al. Intra-articular anesthesia of the distal interphalangeal joint alleviates lameness associated with the navicular bursa in horses. Vet Surg 1997;26:137-140.
- Dabareiner RM, Carter GK, Honnas CM. Injection of corticosteroids, hyaluronate, and amikacin into the navicular bursa in horses with signs of navicular area pain unresponsive to other treatments: 25 cases (1999-2002). J Am Vet Assoc 2003;223:1469-1474.
- Schneider RK. Magnetic resonance imaging evaluation of horses with lameness problems, in Proceedings. 51st Annual American Association of Equine Practitioners Convention 2005;21-34.
- Herthel DJ. Enhanced suspensory ligament healing in 100 horses by stem cells and other bone marrow components, in Proceedings. 46th Annual American Association of Equine Practitioners Convention 2001;319-321.
- Mitchell RD. Treatment of tendon and ligament injuries with UBM powder (Acell), in Proceedings. 14th American College of Veterinary Surgeons Symposium 2004;190-193.
- Caminoto EH, Alves L, Amorim RL. Ultrastructural and immunocytochemical evaluation of the effects of extracorpeal shock wave treatment in the hindlimbs of horses with experimentally induced suspensory ligament desmitis. Am J Vet Res 2005;66:892-895.
- Rose RJ. The treatment of navicular disease-a review and current concepts, in Proceedings. 29th Annual American Association of Equine Practitioners Convention 1984;271-278.
- Matthews NS, Gleed RD, Short EC, et al. Cardiovascular and pharmacokinetic effects of isoxsuprine in the horse. Am J Vet Res 1986;47:2130-2133.
- McIlwraith CW, Frisbie DD, Kawcak CE. Current treatments for traumatic synovitis capsulitis and osteoarthritis, in Proceedings. 47th Annual American Association of Equine Practitioners Convention 2001;180-210.
- Caron JP, Kaneem JB, Miller R. Results of a survey of equine practitioners on the use and perceived efficacy of polysulfated glycosaminoglycan. J Am Vet Med Assoc 1996; 209:1564.
- Crisman MV, Furr MO, Ley WB, et al. Evaluation of polysulfated glycosaminoglycen for treatment of navicular disease, in Proceedings. 39th Annual American Association of Equine Practitioners Convention 1993;219-220.
- Manson RB, Brawher WR, Blaik MA, et al. Oral treatment with cosequin in a double-blinded, placebo-controlled randomized trial as a selective symptom modifying nutraceutical for navicular disease. Vet Ther 2000.
- Eddington ND, White NA. Evidence of the oral absorption of chondroitin sulfate as determined by total disaccharide content after oral and IV administration in horses, in Proceedings. 47th Annual American Association of Equine Practitioners Convention 2001;326-329.
- Triamcinolone, Fort Dodge Animal Health, Fort Dodge, IA 50501.
- Methylprednisolone, Pharmacia & Upjohn, Kalamazoo, MI 49001.
- Aminoglyde, Fort Dodge Animal Health, Fort Dodge, IA 50501.
- Denoix JM. Personal communication, 2006.
- IV Legend, Bayer Corp., Virginia Beach, VA.
- Adequan, Luitpold Pharm Inc., Shirley, NY 11967.
- Cosequin, Nutramax, Baltimore, MD 21234.
|

