Structure and Function of the Equine Digit in Relation to Palmar Foot PainReprinted with permission from the American Association of Equine Practitioners. |
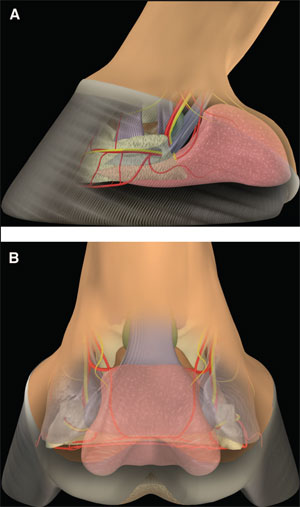 (above) Fig 1. (A) Lateral and (B) palmar schematic representations of the structures of the palmar aspect of the foot. | 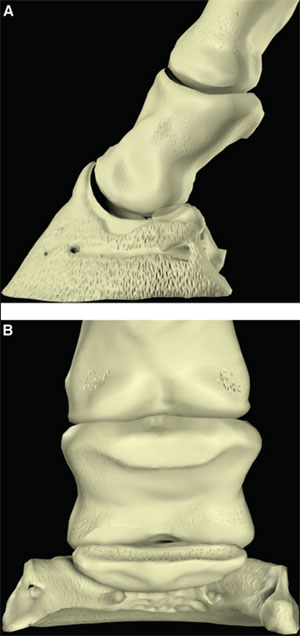 |
| (right) Fig. 2. (A) Lateral and (B) palmar schematic representations of the phalanges. |
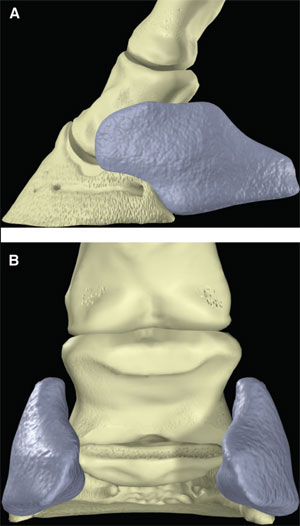 |
| (above) Fig. 3. (A) Lateral and (B) palmar schematic representations of the ungual cartilages. |
2. Anatomy of the Heels
The palmar aspect of the distal phalanx includes the palmar articular surface that articulates with the congruent surface of the middle phalanx and a smaller articular surface perpendicular to the principal joint surface that articulates with the navicular bone (Fig. 2). The flexor surface forms the palmar aspect of the solar surface of the distal phalanx into which the deep digital flexor tendon and distal sesamoidean impar ligament insert. The two palmar processes extend palmarly and abaxially from the body of the bone.1,2 The distal sesamoid or navicular bone is a shuttle-shaped bone with two surfaces; the articular surface articulates with the distopalmar aspect of the middle phalanx, and the secondary smaller articulation with the distal phalanx and a flexor surface forms the distal scutum through which the deep digital flexor tendon passes. It also has two borders - a proximal border to which the collateral sesamoidean ligaments attach and a distal border from which the distal sesamoidean impar ligament arises. It also has two extremities abaxially1,2: the ungual cartilages are rhomboid shaped plates of hyaline cartilage that attach to the palmar process on either side of the distal phalanx such that approximately one-half of their mass is dorsal to the palmar extent of the distal phalanx and one-half of their mass is dorsal to it (Fig. 3). Additionally, these cartilages are approximately one-half within the hoof capsule and approximately one-half proximal to the hoof capsule.
The distal interphalangeal joint is complex and is formed by the articular surfaces of the head of the middle phalanx, distal phalanx, and navicular bone. They form three separate articulations: between the middle and distal phalanx, between the middle phalanx and navicular bone, and between the distal phalanx and navicular bone. However, the articulation between the distal phalanx and the navicular bone moves very little so that the distal phalanx and the navicular bone essentially function as a single articular surface to articulate with the head of the middle phalanx. The two condyles of the head of the middle phalanx, separated by a shallow groove, are congruent with the corresponding surfaces on the distal phalanx and navicular bone. As such, this joint is a hinge that permits flexion and dorsiflexion. The configuration also permits considerable rotation and thus, is also called a saddle joint. Slight mediolateral translocation of the distal phalanx in relation to the middle phalanx may also occur. These movements out of the primary plane of motion allow the foot to temporarily accommodate the irregularities in the ground surface. The shape of the joint capsules reflects the direction of motion within the joint and the position of restraining ligaments (Fig. 4).
The relationship between the three bones is maintained by the collateral ligaments of the distal interphalangeal joint, the collateral sesamoidean ligaments, and the distal sesamoidean impar ligament (Fig. 5). Several other ligaments within the foot attach the ungual cartilage to all three phalanges, the navicular bone, the deep digital flexor tendon, and its contralateral counterpart.3,4
Within the middle of the pastern, the deep flexor tendon is relatively narrow. After passing distally over the middle scutum on the proximopalmar aspect of the middle phalanx, the tendon broadens to pass over the distal scutum formed by the flexor surface of the distal sesamoid bone (Fig. 6). The navicular bursa is interposed between the deep digital flexor tendon and the distal scutum (Fig. 7). The deep digital flexor tendon inserts on the flexor portion of the solar surface of the distal phalanx. The digital flexor sheath encases the deep digital flexor tendon through most of its course in the palmar pastern region and terminates approximately at the level of the middle of the middle phalanx.5,6
The hoof is the integument of the foot. Like skin, the integument of the foot has three layers, namely the epidermis, dermis, and SC tissue. The epidermis is further subdivided into the stratum basale, stratum spinosum, and stratum corneum. The stratum basale and stratum spinosum are frequently referred to collectively as the stratum germinativum. Each of these layers is highly specialized. In brief, the SC tissue forms the digital and coronary cushions and the perichondrium and periosteum of the adjacent surfaces of the distal phalanx and ungual cartilage. The dermis and germinal layer of the epidermis interdigitate to form dermal and epidermal lamellae in the parietal part of the hoof and dermal papillae and epidermal pegs elsewhere. These relate directly to the tubular and lamellar patterns of horn that develop in the stratum corneum. The stratum corneum forms the hoof capsule. The hoof capsule is composed of the wall, sole, frog, and bulbs. The wall of the hoof capsule forms a curved plate that encapsulates the foot from heel to heel; this is thickest at the toe and thinnest at the heels. Inflexions of the walls at the heels form the bars. The sole has a body and two crura, and the overall structure is a concave plate. The crura of the sole abut the walls between the quarters and heels abaxially and the bars and frog axially. The horn of the wall is comparably strong around the circumference of the foot, but because the wall at the heels and quarters are thinner than at the toe, the palmar part of the hoof capsule is more flexible. The digital cushion is a specialization of the SC tissues that underlies the frog. It is bounded medially and laterally by the ungual cartilages, dorsoproximally by the deep digital flexor tendon, palmaroproximally by the more superficial layers of the integument proximal to the coronary band, and distally by the dermis and epidermis of the frog (Fig. 8).7,8
|
|
|
|
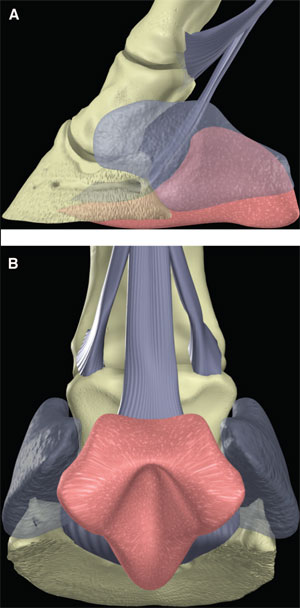 |
| (above) Fig. 8. (A) Lateral and (B) palmar schematic representations of the digital cushion (the ungual cartilages are ghosted) |
3. Function of the Heels
An approximate idea of the function of the equine heels must be pieced together by examination of the gross anatomy of the foot. This helps clinicians form hypotheses regarding function, determine results of functional studies that show the role of the heels in landing and weight bearing, and correlate the conformation with diseased and "healthy" feet. The majority of information available regarding the function of the heels pertains to the forelimbs.
Both macroscopically and microscopically, the very structure of the foot suggests its functions. The classical example is that of frog; the position and shape of it suggest that when pressure is applied, it should cause an increase in pressure in the digital cushion. In turn, this suggests that the increased digital-cushion pressure forces the walls between the quarters and heels abaxially. Alternatively, the position of the frog between the bars and crura of the sole suggests that the frog could also act passively as an expansion joint that permits the heels to expand by some other mechanism. Form per se cannot be used to infer function, but rather, it can be used to formulate hypotheses about function that can be tested with physiological studies.
To understand the function of the heels, it is necessary to explore the kinematics of the stride as it pertains to the foot and the distribution of forces exerted on the structures of the foot. The stride is divided into two primary phases - suspension and stance; the suspension phase involves movement of the limb off the ground, and the stance phase is the period that the limb is in contact with the ground. The stance phase of the stride is subdivided into initial contact, impact, stance, and breakover.9
When moving in a straight line, most horses land on the lateral side of the foot, either at the heel or quarter.10 Landing flat is less common, and landing medially is rare. The foot becomes flat within a few milliseconds and remains so for the duration of the stance phase until the beginning of breakover, which begins the moment the heels leave the ground and ends as the toe leaves the ground. Research suggests that the angle at which the foot initially impacts the ground is determined by proprioceptive feedback that regulates the angle of the distal phalanx to the ground but not the angle of the ground surface of the hoof capsule. Presumably, this works through receptors in the joint capsules and tendons.9
Much of the movement and loading of the distal limb are passive. The distal limb advances during the protraction phase of the stride primarily because of the momentum generated by the proximal limb; the foot is positioned at the appropriate angle for impact. As the limb is loaded by the weight of the horse accelerating toward the ground, the metacarpophalangeal joint dorsiflexes, and the interphalangeal joints flex. The tension in the flexor tendons increases as they lengthen and store energy.11 Because the metacarpophalangeal and interphalangeal joints rotate in opposite directions during loading, the increase in tension in the deep digital flexor tendon that inserts on the distal phalanx is less that that in the superficial digital flexor tendon and suspensory ligament that insert on the proximal and middle phalanges during loading of the limb. However, during the second one-half of the stance phase, the metacarpophalangeal joint moves dorsally and the distal interphalangeal joint also dorsiflexes so that the tension in the portion of the deep digital flexor tendon distal to the insertion of its accessory ligament increases.9 Increased tension in the deep digital flexor tendon causes increase pressure on the navicular bone.12 Additionally, during the normal walk, slight amounts of collateromotion and rotation between the phalanges is normal, primarily at the beginning and end of the support phase of the stride.10
The weight-bearing interaction with the ground is described in terms of the ground-reaction force that is represented as a vector (i.e., it has a magnitude and direction). Additionally, it has a point of action called the center of pressure or point of zero moment. The ground-reaction force vector is divided into three components - vertical, mediolateral horizontal, and craniocaudal horizontal components. Albeit somewhat simplified, the vertical component represents the weight on the limb, and the craniocaudal component is braking (deceleration) and propulsion (acceleration).
The center of pressure is initially at the point of initial contact, and it rapidly moves to the approximate center of the ground surface of the foot where it remains for most of the weight-bearing phase of the stride before it begins to move toward the toe approximately 75% of the way through the stride.13 The force-time curve during the weight-bearing phase of the stride at a trot looks like an inverted U.9 Therefore, the magnitude of the vertical component of the ground-reaction force is low at initial contact and at the end of breakover, and it peaks in the middle of the stride. Thus, at the time when the center of pressure is at the heels, the vertical ground-reaction force is low, and by the time the ground-reaction force is high, it is centered in the ground surface of the foot. However, in addition to the overall force on the limb, the ground-reaction force undergoes high-frequency vibrations during the impact phase that take ~50 ms to disappear.9,14 It is these oscillations associated with impact in the ground-reaction force that are thought to be the greatest source of injury in equine and human athletes.
It has been known for a long time that the hoof expands as it is loaded during weight bearing. Maximal expansion of the foot occurs during ~33% of the overall stance phase of the stride. This occurs after the oscillations of impact have subsided and the center of pressure is near the middle of the ground surface of the foot but before the vertical component of the ground reaction force is maximal.15 The width of the heels has return to preexpansion dimensions by 75% of the stance-phase duration after which the heels contract to coincide with the movement of the center of pressure toward the toe.15 It has also been shown in vitro that the majority of the dampening of the vertical component of the ground-reaction force occurs between the hoof and the distal phalanx.16 Thus, the expansion of the palmar one-half of the foot seems to be related to the absorption of the energy from the vertical component of the ground-reaction force before the rate of loading tapers off in the middle of the stance phase of the stride; however, the mechanism by which the foot expands is incompletely understood. Traditional hypotheses suggest that the pressure in the digital cushion is increased either by descent of the middle phalanx toward the digital cushion or by upward pressure from the frog as it contacts the ground; this increase in pressure is thought to force the abaxial movement of the hoof. Research studies have provided either inconclusive or conflicting results. One showed no consistent relationship between frog pressure and hoof expansion.17 Another showed increased hoof expansion with increased frog pressure, but the hoof expanded to a lesser extent when no frog pressure was present.15 A third study showed that the pressure in the digital cushion dropped during weight bearing, which suggests that foot expansion was not dependent on frog pressure and that it was the expansion of the hoof by some other mechanism that caused the drop in frog pressure.18
There are significant differences in the landing of the horse, the movement of articulations, and the distribution of stress within the distal limb when a horse turns in a tight circle compared with when a horse moves in a straight line.19 When a horse moves in a tight circle, the limb to the inside of the circle is abducted before contacting the ground. The foot is more likely to land flat or toe first. The load, as evidenced by increased strains in the hoof wall at the lateral quarter and reduced strains in the medial quarter, moves toward the lateral wall.20 As the limb is retracted during the stride, the distal interphalangeal joint rotates medially during the stance phase. This places considerable stress on the collateral ligaments of the distal interphalangeal joint and the suspensory ligaments of the navicular bone.
The evidence from the aforementioned studies suggests that the principle function of the heels is to dissipate energy during the impact phase of the stride. Therefore, it follows that any conformation or imbalance that impairs this natural function is likely to result in disease, because the majority of lameness associated with the heels are related to stress-induced injuries. Stress-induced injury is both a function of the structure of the heel and the severity of work performed. That is, injury may occur in any horse if the insult is severe enough, but if there is an anatomical pre-disposition because of abnormal balance or conformation, or a farriery manipulation that focuses stress, then injuries will be more likely to occur at submaximal work. If disease is the result of abnormal stresses and impaired function and poor structure pre-disposes the horse to disease, it follows that comparison of structures of the feet of horses with and without disease should indicate what good conformation is and have implications for function.
There are many assumptions about what constitutes a healthy foot based on clinical experience, and the characteristics that are generally considered desirable usually relate to the surface structure of the foot. These characteristics include a straight foot pastern axis, short upright heels, a foot that is approximately symmetrical about the frog, and a foot that is about as wide as it is long. Additionally, the width of the frog at its base (i.e., its widest and palmar-most aspect) is approximately one-half to two-thirds of its length.
Examination of the palmar one-half of the foot shows that the internal structures are similar between horses at skeletal maturity, regardless of breed or type.21 Some horses feet continue to develop, probably under the influence of some stimulation related to exercise and weight bearing, whereas others do not. It is the former category of internal development that correlates with the external appearance of the archetypal "healthy" foot. Such horses develop thicker ungual cartilages with fibrocartilagenous extensions that merge into the digital cushion, and the digital cushion contains more fibrocartilage. The thickened ungual cartilages contain numerous foramina of which each contains a central vein and a substantial microvascular network that includes numerous vascular anastomoses. The digital cushions that contain fibrocartilage are better at absorbing energy than those that contain predominantly fat and elastic tissue. The elaborate network of veins and microvasculature associated with the axial aspect of the ungual cartilages is compatible with a hydrodynamic mechanism for dampening the stresses associated with the impact phase of the stride. In contrast, "unhealthy" feet are associated with thin ungual cartilages with few to no vascular foraminae and digital cushions primarily composed of fat and elastic tissue. This provides both additional evidence for the impact-dampening function of the heels and a potential hydrodynamic mechanism for it.21
4. Conformation, Balance, and Shoeing
The definitions of conformation and balance in relation to the equine foot are frequently ambiguous. Therefore, for the context of this review, the author considers the term conformation to refer to the shape of the limb, and as such, it refers strictly to the limb at rest (if the hoof is excluded, it changes little and slowly in adult horses). In contrast, the author considers the term balance to refer to the way the hoof relates to the underlying structures in the foot and to the limb proximal to the coronary band with respect to its position, size, and shape as well as the way the hoof relates to the ground both at rest and in motion. Because the hoof is constantly growing and because of the viscoelastic nature of the hoof capsule, foot balance can change rapidly in adult horses. Few studies have examined the effect of conformation with a "normal hoof" on foot function. One such study examined the relationship between slope of the foot-pastern axis and the distribution of force during the weight-bearing phase of the stride; a higher percentage of weight is borne by the palmar one-half of the foot in horses with sloping foot-pastern axes compared with horses with upright foot-pastern axes.22 The implication is that the dorsal structures are more prone to concussion if the pastern axis is upright and vice versa.
There are many variables associated with balance that may influence the vibrations during the impact phase of the stride or the magnitude and position of the ground force; our knowledge about how these impact the function and structural integrity of the heels is far from complete. However, intuitively it would seem that any particular pattern of conformation or balance that either increases the vibrations associated with initial impact or moves the point of force palmarly during the weight-bearing phase of the stride has the potential to damage the structural integrity of the palmar one-half of the foot and thus, alter function.
The influences of balance and shoeing on foot function have both been examined extensively. To alter the angle of the hoof to change either dorsopalmar or mediolateral imbalance, a wedge pad is applied; because a wedge pad is a shoeing manipulation, this aspect of shoeing is considered in conjunction with balance. Probably no external manipulation has been examined more than the effects on foot function of altering the angle of the hoof, particularly elevating the heels, because it is a time-honored method of treating horses with heel pain, and the simplest way to study the effects of altered dorsopalmar hoof balance. At rest, elevating the heels causes the interphalangeal joints to flex (the distal joint more so than the proximal one) and the metacarpophalangeal joint to dorsiflex modestly.23 The opposite response occurs if the toe is elevated. The center of pressure remains in approximately the same position if the heels are elevated13,24; however, it has been pointed out that this results in a small ground-surface area that theoretically could lead to injury, because the same force is distributed over a smaller area.25 The clinical observation that elevating the heels decreases the growth of the wall at the heels supports this contention, because wall growth is generally inversely related to load. Elevating the heels decreases the tension in the deep digital flexor tendon.26 It also increases the intra-articular synovial fluid pressure.27 Additionally, silicone-casting studies of the distal interphalangeal joint show that the contact between the head of the middle phalanx and the articular surfaces of the distal phalanx and navicular bone is no longer as even with heel elevation; the contact is concentrated more dorsally.27
In motion, elevating the heels increases the likelihood of heel-first contact and causes the distal phalanx to roll forward more after first contact before the foot becomes in stable contact with the ground.28,29 During the second one-half of the stance phase of the stride, elevating the heels delays the movement of the center of pressure to the toe at breakover and thus, their unloading.13 Additionally, either elevating the toe or the heel is reported to increase the impulse (area under force X time curve) during the total weightbearing phase of the stride.28 It has little impact on motion within the distal interphalangeal joint outside the saggital plane.29
It has long been held that the foot-pastern axis should be straight (i.e., 180°), but the evidence to confirm that this is the least injurious is limited. However, the evidence of optimal congruency of the contact between the middle phalanx and the distal phalanx and navicular bone and the lowest impulse during motion with a straight foot-pastern axis support this concept. Another clinical feature considered indicative of poor dorsopalmar balance, namely collapsed heels, has recently been shown to be unrelated to the force applied to the navicular bone; however, the angle of the solar margin of the distal phalanx to the ground does correlate with the force on the navicular bone.30
The effect of changing mediolateral balance has been investigated in both stationary and moving horses in a similar fashion to dorsopalmar balance by adding medial or lateral wedges to a foot. Elevating one-quarter to induce imbalance causes rotation, collateromotion, and sliding between the phalanges.31,32 It also shifts the landing pattern and the center of pressure during the stance phase of the stride toward the elevated side.12 Logically, this would induce stresses in the collateral and collateral sesamoidean ligaments as well as the articular surfaces themselves. The challenge, of course, is to relate these deeper structural changes to superficially detectable changes. Optimal staticmediolateral balance based on external landmarks has been related to symmetry of the hoof and distal limb; however, radiography is a useful if not indispensable adjunct to assessing mediolateral balance. As such, mediolateral balance has been related to both parallelism of the articular surface of the distal phalanx with the ground and parallelism of the articular surfaces of the distal and proximal interphalangeal joints.33 It is hard to imagine a beneficial situation in which the articulations are uneven, so the latter takes precedence over the former.
Steel shoes attached with nails are known to diminish the natural dampening mechanisms of the distal limb compared with unshod feet.14 Specifically, shoeing with plain steel shoes increases the frequency and amplitude of the oscillations in the ground-reaction force during the impact phase of the stride and decreases the expansion of the quarters and heels during the stance phase. Certain plastic shoes used with synthetic viscoelastic pads reduce both the frequency and magnitude of the vibrations associated with the foot's impact with the ground.14 Additionally, finite element analysis suggests that the nails, particularly those placed most palmarly in the foot, cause concentration of stress in the wall.34 Egg-bar shoes, which function as a short palmar extension of the shoe, are probably the next most frequently used shoe for the treatment of heel pain. Studies have primarily examined the effect of egg-bar shoes when the horse is standing or moving on a firm, flat surface. At rest, they move the center of pressure palmarly in relation to the toe; this is considered beneficial for the deep digital flexor tendon and navicular bone but is potentially harmful to the hoof capsule at the heels.24 Extreme heel extension combined with elevation, as seen in the use of Patten shoes that are used to treat horses with deep digital flexor tendon injuries, shortens/deforms the heels; at the same time, it causes the toe to lengthen and become more convex and the sole to become more concave.
5. Conclusion
The equine foot is an intricately designed structure that accommodates the weight-bearing and propulsive functions of the foot at rest and during locomotion. It seems that the function of the various structures of the heels varies with the phase of the stride. At impact, they dampen the damaging high frequency of the rapidly accelerating and decelerating forces. During the stance phase of the stride, they facilitate the expansion of the hoof as increasing weight is borne by the foot and the flexion of the interphalangeal joint. At breakover, they participate in the extension/dorsiflexion of the distal interphalangeal joint. Consequently, it follows that if the heels are unduly loaded during the stance phase of the stride because of either poor conformation or improper farriery practices, then the additional load is likely to cause injury, damage these structures, and impair their function. During the second onehalf of the stride, particularly at breakover, the navicular apparatus is under the greatest amount of stress. Consequently, it follows that either poor conformation or farriery practices that increase these stresses are injurious to the navicular bone, its associated ligaments, and the deep digital flexor tendon.
References
Getty R. Equine osteology: the digit of the manus. In: Getty R, ed. Sisson and Grossman's the anatomy of the domestic animals, 5th ed. Philadelphia: W.B. Saunders, 1975;291-296.
Nickel R, Schummer A, Wille K-H, et al. Bones of the thoracic limb of the horse. In: Nickel R, Schummer A, Seiferle E, et al., eds. The anatomy of the domestic animals. Berlin: Verlag Paul Parey, 1986;71-74.
Sisson S. Equine syndesmology: articulations of the manus. In: Getty R, ed. Sisson and Grossman's the anatomy of the domestic animals, 5th ed. Philadelphia: W.B. Saunders, 1975;355-362.
Nickel R, Schummer A, Wille K-H, et al. Digital joints of the horse. In: Nickel R, Schummer A, Seiferle E, et al., eds. The anatomy of the domestic animals. Berlin: Verlag Paul Parey, 1986;197-201.
Sisson S. Equine myology: fasciae and muscles of the forearm and manus. In: Getty R, ed. Sisson and Grossman's the anatomy of the domestic animals, 5th ed. Philadelphia: W.B. Saunders, 1975;424-431.
Seiferle E, Frewein J. Muscles of the digits. In: Nickel R, Schummer A, Seiferle E, et al., eds. The locomotor system of domestic animals. Berlin: Verlag Paul Parey, 1986;386-391.
Stump JE. Anatomy of the normal equine foot, including microscopic features of the laminar region. J Am Vet Med Assoc 1967;151:1588-1598.
Habermehl K-H. The digital organ of the horse. In: Schummer A, Wilkens H, Vollmerhaus B, et al., eds. The circulatory system, the skin, and the cutaneous organs of the comestic mammals. Berlin: Verlag Paul Parey, 1981;541-557.
Clayton HM. Effects of hoof angle on locomotion and limb loading. In: White NA, Moore JN, eds. current techniques in equine surgery and lameness, 2nd ed. Philadelphia: W.B. Saunders Company, 1998;504-509.
Chateau H, Degueurce C, Denoix JM. Evaluation of threedimensional kinematics of the distal portion of the forelimb in horses walking in a straight line. Am J Vet Res 2004;65: 447-455.
Wilson AM, McGuigan MP, Su A, et al. Horses damp the spring in their step. Nature 2001;414:895-899.
Wilson AM, McGuigan MP, Fouracre L, et al. The force and contact stress on the navicular bone during trot locomotion in sound horses and horses with navicular disease. Equine Vet J 2001;33:159-165.
Wilson AM, Seelig TJ, Shield RA, et al. The effect of foot imbalance on point of force application in the horse. Equine Vet J 1998;30:540-545.
Benoit P, Barrey E, Regnault JC, et al. Comparison of the damping effect of different shoeing by the measurement of hoof acceleration. Acta Anat 1993;146:109-113.
Roepstorff L, Johnston C, Drevemo S. In vivo and in vitro heel expansion in relation to shoeing and frog pressure. Equine Vet J 2001;(Suppl):54-57.
Willemen MA, Jacobs MW, Schamhardt HC. In vitro transmission and attenuation of impact vibrations in the distal forelimb. Equine Vet J 1999;30(Suppl):245-248.
Colles CM. The relationship of frog pressure to heel expansion. Equine Vet J 1989;21:13-16.
Dyhre-Poulsen P, Smedegaard HH, Roed J, et al. Equine hoof function investigated by pressure transducers inside the hoof and accelerometers mounted on the first phalanx. Equine Vet J 1994;26:362-366.
Chateau H, Degueurce C, Denoix JM. Three-dimensional kinematics of the equine distal forelimb: effects of a sharp turn at the walk. Equine Vet J 2005;37:12-18.
Thomason JJ. Variation in surface strain on the equine hoof wall at the midstep with shoeing, gait, substrate, direction of travel, and hoof shape. Equine Vet J 1998;(Suppl):86-95.
Bowker RM, Van Wulfen KK, Springer SE, et al. Functional anatomy of the cartilage of the distal phalanx and digital cushion in the equine foot and a hemodynamic flow hypothesis of energy dissipation. Am J Vet Res 1998;59:961-968.
Barrey E. Investigation of the vertical hoof force distribution in the equine forelimb with an instrumented horseboot. Equine Vet J 1990;(Suppl):35-38.
Bushe T, Turner TA, Poulos PW, et al. The effect of hoof angle on coffin, pastern and fetlock joint angles, in Proceedings. 33rd Annual American Association of Equine Practitioners Convention 1987;729-738.
Rogers CW, Back W. Wedge and eggbar shoes change the pressure distribution under the hoof of the forelimb in the square standing horse. J Equine Vet Sci 2003;23:306-309.
Balch O, White K, Butler D. Factors involved in the balancing of equine hooves. J Am Vet Med Assoc 1991;198:1980-1989.
Lochner FK, Milne DW, Mills EJ, et al. In vivo and in vitro measurement of tendon strain in the horse. Am J Vet Res 1980;41:1929-1937.
Viitanen MJ, Wilson AM, McGuigan HR, et al. Effect of foot balance on the intra-articular pressure in the distal interphalangeal joint in vitro. Equine Vet J 2003;35:184-189.
Balch OK. The effects of changes in hoof angle, mediolateral balance, and toe length on kinetic and temporal parameters of horses walking, trotting and cantering on a high-speed treadmill: Washington State U 1993;21-30.
Chateau H, Degueurce C, Denoix JM. Effects of 6 degree elevation of the heels on 3D kinematics of the distal portion of the forelimb in the walking horse. Equine Vet J 2004;36: 649-654.
Eliashar E, McGuigan MP, Wilson AM. Relationship of foot conformation and force applied to the navicular bone of sound horses at the trot. Equine Vet J 2004;36:431-435.
Caudron I, Grulke S, Farnir F, et al. Radiographic assessment of equine interphalangeal joints asymmetry: articular impact of asymmetric bearings (Part II). Zentralbl Vet Med A 1998;45:327-335.
Chateau H, Degueurce C, Jerbi H, et al. Three-dimensional kinematics of the equine interphalangeal joints: articular impact of asymmetric bearing. Vet Res 2002;33:371-382.
Caudron I, Miesen M, Grulke S, et al. Clinical and radiological assessment for corrective trimming in horses. J Equine Vet Sci 1997;17:375-379.
Hinterhofer C, Stanek C, Haider H. The effect of flat horseshoes, raised heels and lowered heels on the biomechanics of the equine hoof assessed by finite element analysis (FEA). J Vet Med A Physiol Pathol Clin Med 2000;47:73-82.